 |
Eager Space | Videos by Alpha | Videos by Date | All Video Text | Support | Community | About |
|---|


SpaceX has caught a massive rocket. So what's next?
https://arstechnica.com/space/2024/10/spacex-has-caught-a-massive-rocket-so-whats-next/#gsc.tab=0
Boca Chica Draft Programmatic Environmental Assessment for Starship/Super Heavy
https://web.archive.org/web/20211001032259/https://www.faa.gov/space/stakeholder_engagement/spacex_starship/media/Draft_PEA_for_SpaceX_Starship_Super_Heavy_at_Boca_Chica.pdf

Welcome to eager space...
There has been a fair bit of discussion about propellant supply for starship recently.

If you've watched my videos and especially if you follow me on TwiX, you know that I'm a fan of Eric Berger. I pay for Ars Technica to support his writing there and I own both of his books, liftoff which covers Falcon 1 and Reentry which covers Falcon 9.
And I'm therefore happy to find a case where I disagree with him.
On October 28th, he wrote a nice article entitled "SpaceX has caught a massive rocket. So what's next", an article that I mostly agree with. As always, you can find a link to references in the video description.
There's a little section on propellant supply that caught my eye and aligned with a topic that I was already going make a video on.
Here's what he wrote: (read)
Is this going to be a significant problem for Starship?

Hmm...
I'm going to start with a couple of questions...
The first is "would we expect an existing industry to already support a major new use of a commodity?"
In a free market economy, it would be very surprising if the answer to that question was "yes", for the simple reason is that if it were true, the companies who sell that commodity would have a lot of excess capacity and excess capacity costs money.
So I think I'm going to have to label the comparison of starship to existing supplies as an exercise in hyperbole, which is something that I certainly would never do.
The deeper question is whether liquid oxygen availability will be a significant barrier to missions to the moon and Mars? And does SpaceX need to do something themselves to deal with this?
And what about liquid methane?
That is our subject for today.
And I'm going to use the 4 launches per day flight rate that Berger mentioned. It's far more than you need for a lunar mission but it seems like a pretty ridiculous number and Starship is a pretty ridiculous rocket.
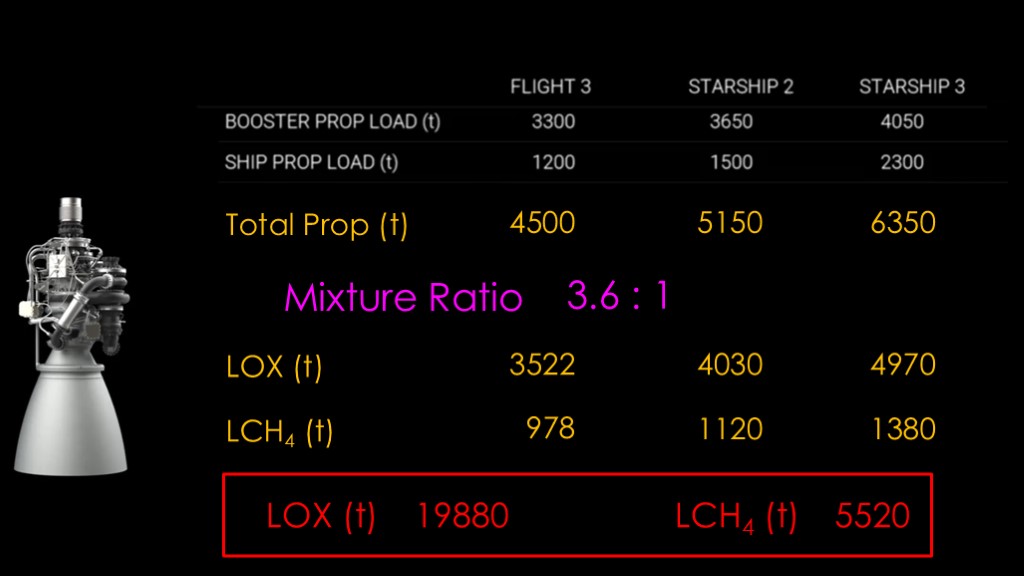
Our first task is to understand how big the problem is, and SpaceX has nicely given us some great data. The total propellant load is 4500 tons for starship version 1, 5150 tons for variant 2, and 6350 tons for block 3.
That is total propellant - both liquid oxygen and liquid methane. We need to know how much of each.
To do that, we need to know the mixture ratio for the engine. That mixture ratio is mostly set by the chemistry of our propellants, and Raptor runs at about 3.6 to 1 as most methane engines do. That is 3.6 parts liquid oxygen by weight to 1 part liquid methane, or 78% liquid oxygen and only 22% liquid methane. You can find the mixture ratio for most engines in their Wikipedia article, and if not, you can pick an engine that uses the same propellant mix; you'll be close enough for this sort of thing.
Knowing that, we can come up with the following numbers.
We'll use the numbers for starship block 3 and will multiply them by four to get to four launches a day, and that gives us 19880 tons of liquid oxygen and 5520 tons of liquid methane.
Assume for the moment that we have suppliers that can produce that much - can we get it to the launch site?
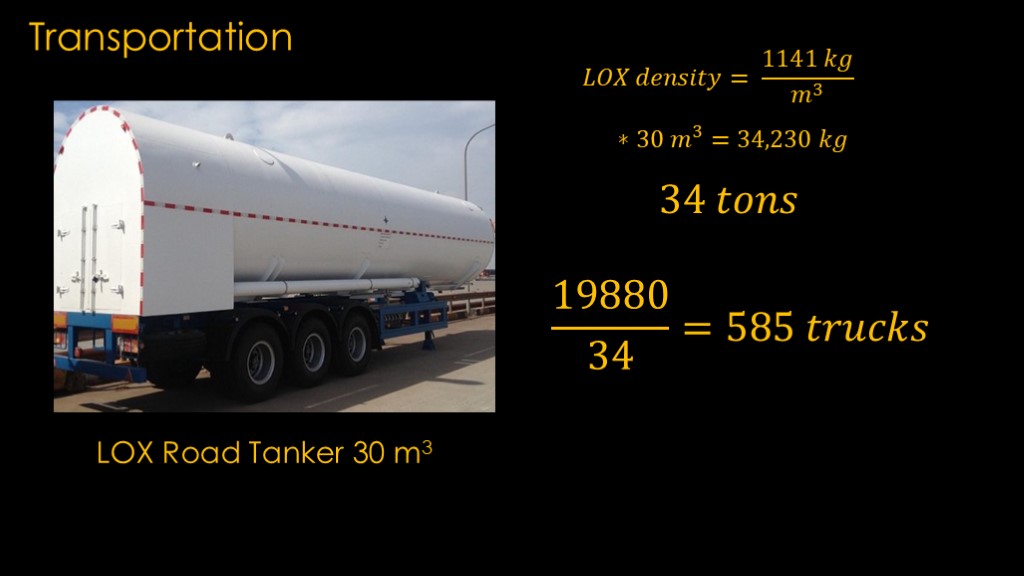
Liquid oxygen transportation requires a special tanker truck, and this one can carry 30 cubic meters of liquid oxygen.
The density of liquid oxygen is 1141 kilograms per cubic meter. Multiply that by 30 cubic meters and we get 34,230 kilograms, which I'm just going to call 34 tons.
Our target capacity is 19880 tons, and at 34 tons per truck, that means we need 585 liquid oxygen trucks for four launches. That seems like a lot of trucks to send down a two lane highway to the launch site.

In yet another "I love the internet" moment, the Texas department of transportation can help, with this map from the boca chica area.
https://experience.arcgis.com/experience/6c0166bfc5144afe83926a3a529a8d03
On the left we have the Starbase complex, and on the right there is the launch pad area.
And in the middle there's a little number which is the traffic count, and it says "3067", which is the vehicle count per day. Assuming that count is the total both ways, adding 585 truck trips would be a 38% increase in traffic.
That sounds like a lot, but it's a small number for a two-lane highway.
If we assume each launch stops propellant transportation for 2 hours, that gives us 16 hours to handle the trucks. 585 trucks in 16 hours is roughly one truck every 100 seconds.
That doesn't seem like a lot of traffic.
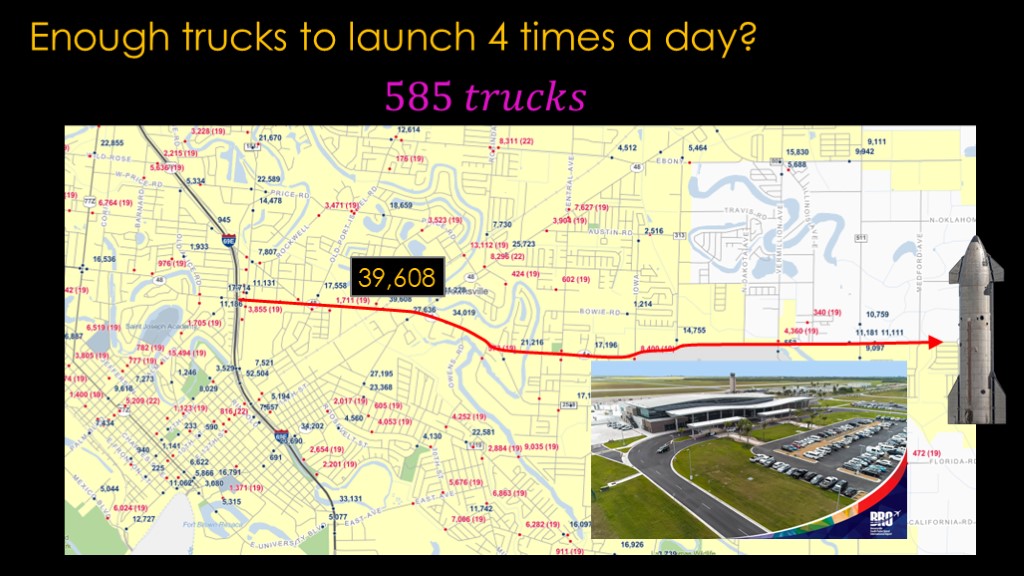
Unfortunately, we're looking at the empty part of the highway.
If we head back towards civilization, we can trace the boca chica highway back to the closest major freeway. On that route, there are pretty close to 40,000 vehicles per day.
Add 585 round trips to that, and it's only a 3% increase in vehicle trips, but that's a pretty busy road, a road that unfortunately goes past the Brownsville south padre island airport.
So, maybe you can do it, but you may not make the natives very happy.

What about unloading all the trucks? It takes something like 30 minutes to unload a cryogenic tanker, which gives us a capacity of 32 trucks per 16 hour day. We would need 18 stations operating in parallel to make that work, but there are many warehouses with far more loading bays.
This all seems possible. Assuming SpaceX can find suppliers willing to make more liquid oxygen, Texas government is okay with the traffic, and SpaceX builds the delivery center, they should be able to support four launches a day.

But there's something missing.
We need to think about the methane.

The amount of liquid methane we need is a lot less than the amount of liquid oxygen, but it's important to remember that the density of liquid methane is only 442 kilograms per cubic meter, and that means only 13 tons per truck, and 425 trucks for four launches a day.
The low density of the liquid methane means the truck count is higher than expected.
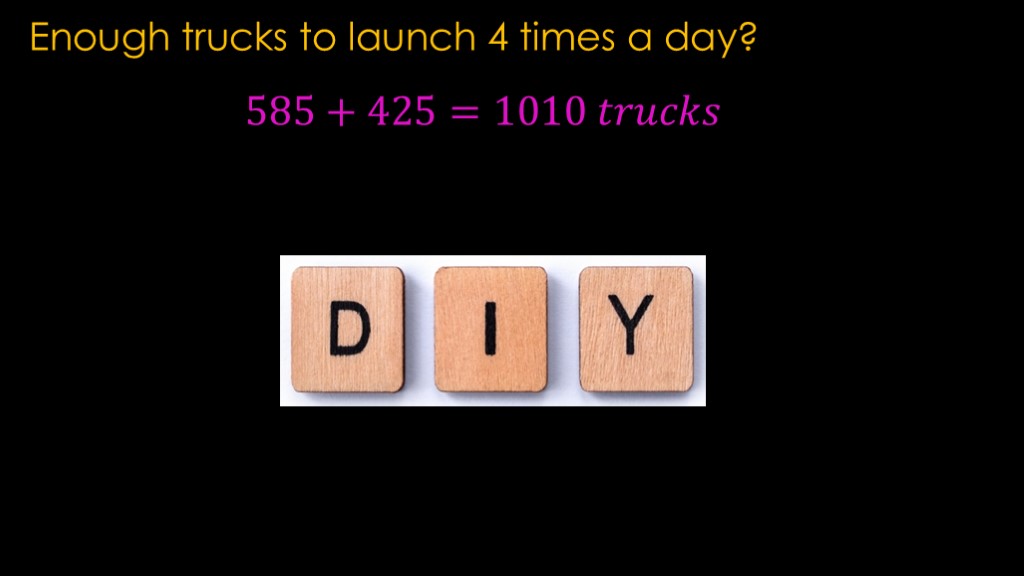
Redo our calculations, and we need just over a thousand trucks a day to launch four times a day.
The road capacity is there. You would need to figure out the production and logistics side, but I believe it's possible.
It is a lot of complexity from an operational standpoint. It would certainly be more convenient if you could create the liquid oxygen you need on-site.

To start, we need to understand how liquid oxygen is created.
Conceptually, it's pretty simple - we extract oxygen from the air, cool it down very cold, and we get liquid oxygen. Since starship is a very big rocket, we need a very big refrigerator, and then we're golden.
That's the concept, though it needs to be a special kind of refrigerator.
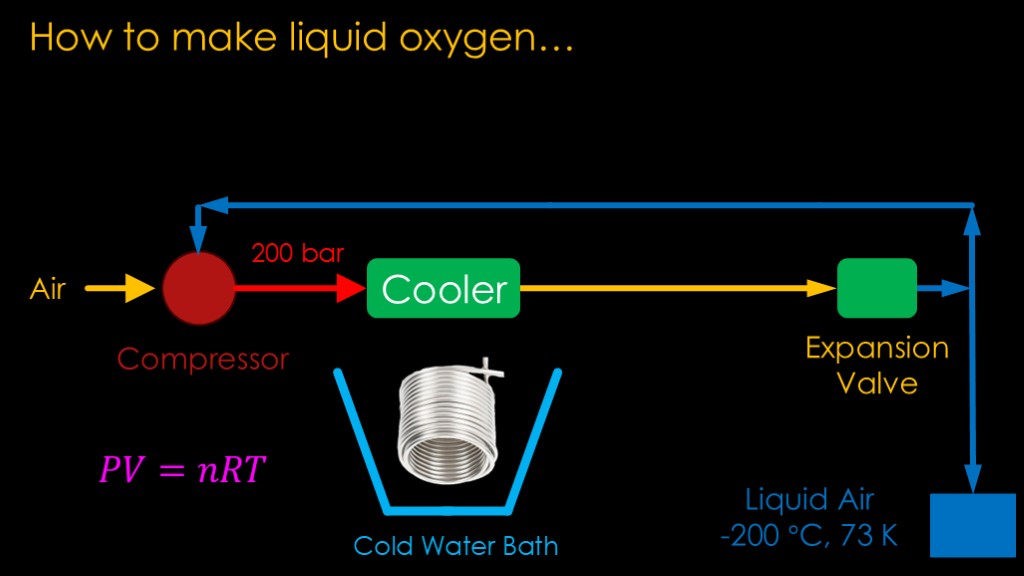
We start with a standard refrigeration approach.
We take air and run it through a compressor that bumps it up to a very high pressure - say something around 200 times atmospheric pressure, or 200 bar. Remembering the Ideal Gas law, if we increase the pressure of a gas, we see a rise in temperature.
The pressure is quite high so we will see quite a bit of jump in temperature. That hot air is then run through a cooler to cool it down. In this case the cooler is likely a coil of tubing in an a cold water bath that is pretty close to freezing. That cold water bath has a conventional refrigeration system to keep it cool.
That cooled high pressure air is then allowed to expand in an expansion valve, and because the pressure drop is high, the temperature goes way down.
Some of the cooled air gets cold enough to condense out as liquid air, at about -200 degrees celcius or 73 kelvin
The rest of the cooled air is fed back into the compressor for another cycle.
This is pretty much how your fridge works, except your fridge has a closed system using a refrigerant that works better than air, and the cooler part is typically a set of coils on the back of the fridge that are cooled by air.
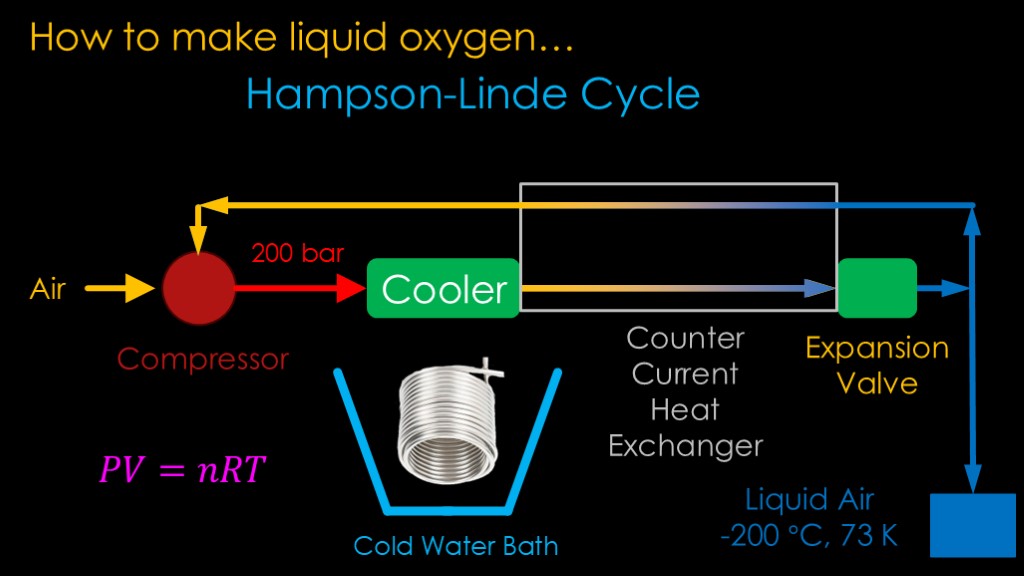
This approach works but it's hard to get cold enough to get much liquid air. What we need is a way to further reduce the temperature of the chilled gas before it hits the expansion valve. That is done by a clever device known as a counter current heat exchanger.
The return gas from the expansion valve and the cooled gas from the cooler run next to each other in opposite directions through a heat exchanger. That heats the gas coming back from the expansion valve while cooling the gas from the cooler.
This makes a big difference in the amount of liquid air that can be generated.
The approach is known as the Hampson - Linde (Hampson lin-da) cycle.

We now have a container of liquid air at 73 kelvin. It's about 78% nitrogen, 21% oxygen, and 1% Argon. We need a way to separate them.
And here we get a bit lucky with how the physics works out. We can plot the liquification temperatures of oxygen, argon, and nitrogen on this kelvin temperature scale.
Liquid nitrogen cannot exist at a temperature higher than 77K, so if we heat up our liquid air to about 80 K, the nitrogen will boil off.
Our liquid air is now just the oxygen and argon.
We can play the same trick again. The boiling point of Argon is about 87 Kelvin, and we can heat up our liquid air about 88.5 kelvin and extract the argon that boils off. This a lot harder to do, because the boiling point of oxygen is only 3 degrees higher than argon so it's tougher to boil off all the liquid argon without losing any liquid oxygen.
In the end, all that is left is the liquid oxygen component.
This process is known as fractional distillation and is a very common processing technique.
The very cold gaseous nitrogen can be used to pre-chill the air coming into the process, it can be re-chilled to produce liquid nitrogen that we can use or sell.
But if we are generating thousands of tons of liquid oxygen, we are generating a lot more liquid nitrogen than we want.
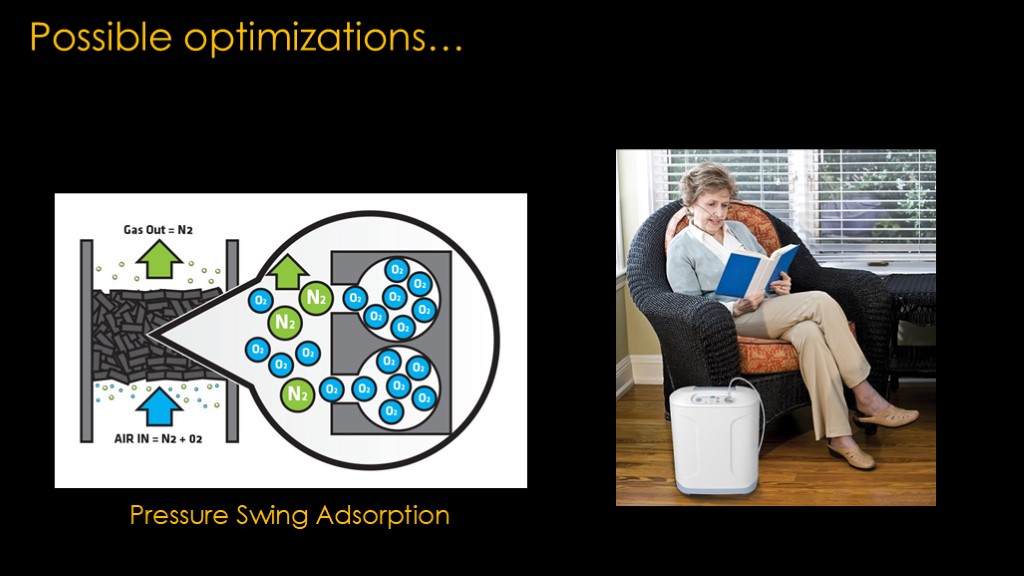
It would be nice if we could increase the percentage of oxygen that we feed into our chiller so we spend less effort chilling nitrogen that we don't want.
One such process to do that is called pressure swing adsorption. You use an material that has affinity for one molecule over another - in this case a carbon molecular sieve with pores that can hold oxygen but not nitrogen - and you put pressurized air into it.
The oxygen in that air is pushed into the molecular sieve and the nitrogen passes through.
Then you release the pressure and the oxygen that is hanging out in the molecular sieve comes out.
You therefore get one chunk of output that is very high in nitrogen followed by one that is very high in oxygen. Toss away the nitrogen batch, and feed the oxygen batch into your liquid oxygen plant. You could also do this to remove argon.
This seems like advanced technology, but it has been used in medical oxygen concentrators for quite some time, providing a continuous flow of oxygen at a concentration of 90-95%. It's possible to go higher with multiple stages.
There is good technology for creating liquid oxygen.

air-liquide-e-c-standard-plants-september-2017.pdf
Now comes the hard question. How will we build a liquid oxygen factory?
It would probably be good to choose a company that already uses the technology. A company like Air Liquide with a long history of supplying liquid gases for rockets.
They happen to already have a product named Yango that can produce 330-770 tons of liquid oxygen per day.
We need about 26 times that amount of liquid oxygen to launch 4 times a day, but that's a matter of scale rather than technology.

We would need to power such a plant. Yango takes about 400 kilowatt hours to produce a ton of liquid oxygen, so the total for 4 launches would be 8 gigawatt hours.
That's over a 24 hour period, so it's a constant power input of 333 megawatts. That's a lot of power.

You can think of the liquid oxygen plant as being roughly equivalent to 3 Tesla gigafactories running full out.

But it's not an unprecedented amount of power. Large aluminum smelters can use up to 2400 megawatts of power.

And now it's time to talk about methane. But first...

Choose wisely...
Like and subscribe, or
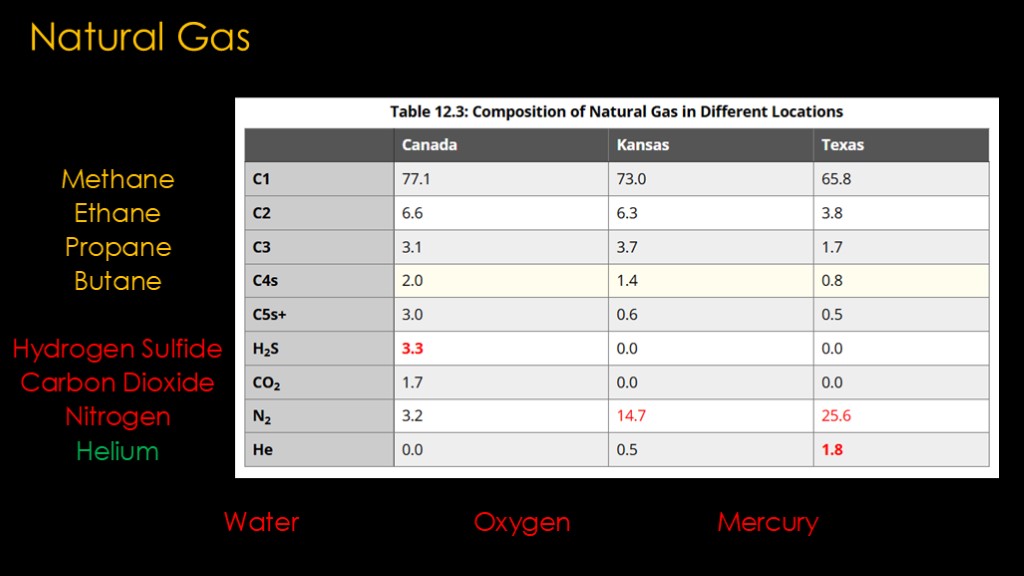
Natural gas is the most common source of methane
Natural gas is, well, "natural", and what comes out of the ground has a lot of things in it and exactly what is in it differs depending on where it comes from.
The most common components are what are known as alkanes, classified by the number of carbon molecules they contain. The most common ones being methane, ethane, propane, and butane.
There are a bunch of other components of the mix. Some are things we don't want - hydrogen sulfide, carbon dioxide, and nitrogen. The last component is helium - it's not well known that helium comes from fossil fuel sources and that's pretty much our only source for it.
There are other things to be concerned about - water, oxygen, and mercury are common.
If you hear a rocket company say, "our engines run on natural gas", this isn't the kind of gas they are talking about.

To get rid of the things we don't want, the natural gas needs to be processed in a plant like this one.
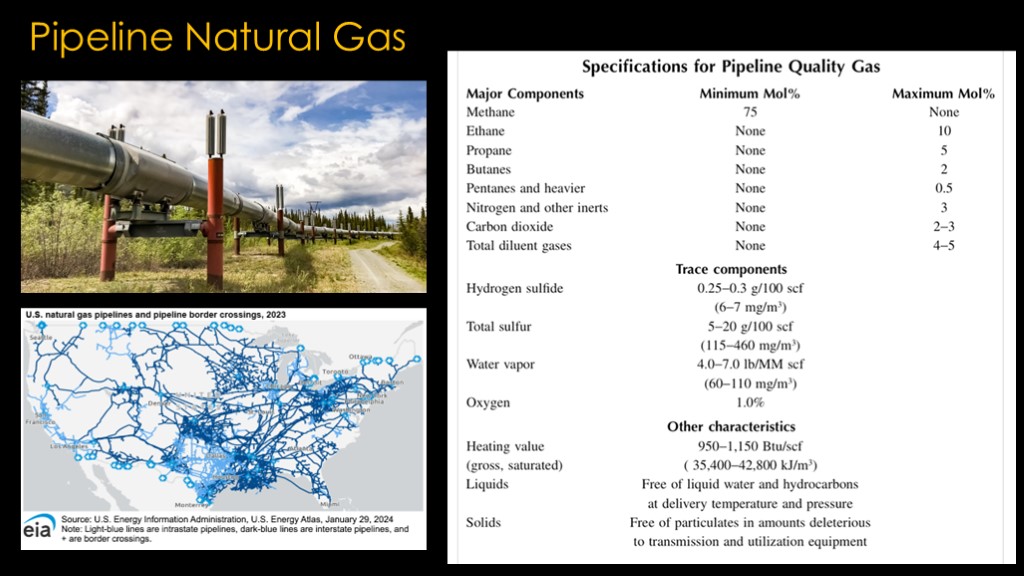
The processing plant gives us pipeline natural gas, which is what we mean when we talk about natural gas.
We still aren't to the point that this is useful in rocketry - note that it contains up to 5% carbon dioxide and nitrogen, and it is a gas and we need a liquid.

Cooling pipeline natural gas gives us liquid natural gas or LNG, though that is often done at the source of the natural gas as part of the purification process instead of as two discrete steps.
This increases the density significantly and makes it much easier to ship from one place to another.
That's a liquid. Is it used for rocket fuel?

The answer is pretty clearly yes.
Blue Origin says their BE-4 engine uses liquified natural gas.
ULA tells us the same on the BE-4 fact sheet for their Vulcan rocket.
But the answer is also no.
Question: Tory Bruno, will you be using pure methane with the BE-4 or will it be a mix closer to what we known as LNG?
Answer: Methane
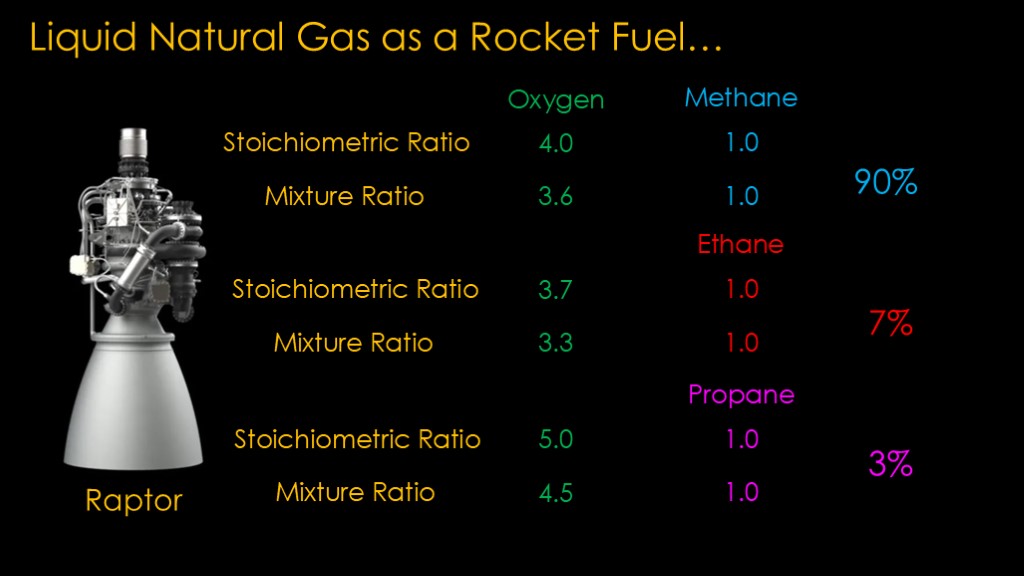
The problem is liquid natural gas is simply natural gas that has been liquified. It could be pipeline gas, or it could be 99.9% methane - as far as I can tell, there's no standard - it's all based on whether you can burn it without messing up your equipment.
Could we use pipeline gas processed so it just consists of methane, ethane, and propane as rocket fuel?
I see a couple of problems.
The first is mixture ratio.
The stoichiometric ratio for oxygen and methane is 4 parts oxygen to 1 part methane by mass - that's the amount of each we would put together to get full combustion.
Rockets almost always run fuel rich because the stoichiometric ratio leads to combustion that is too hot and it's far better to have extra hot fuel than extra hot oxygen because very hot oxygen likes to eat... everything...
Raptor therefore has a mixture ratio of 3.6 to 1 - with a little extra fuel - though I'll note here that I expect that SpaceX dynamically controls the mixture ratio during different parts of flight.
If we are burning ethane instead of methane, the chemistry is different and the stoichiometric ratios is about 3.7 to 1. That means that we probably want a mixture ratio of around 3.3.
Propane also has a different chemistry, with the stoichiometric ratio of 5 to 1 and a probable mixture ratio around 4.5 to 1.
We fuel our rocket with 90% methane, 7% ethane, and 3% propane. What mixture ratio should we use?
I don't know, but I do know having a combination of these three fuels will change the mixture ratio that we want to use, and that change will depend on the proportion of each fuel. And if you get it wrong your engines might melt.
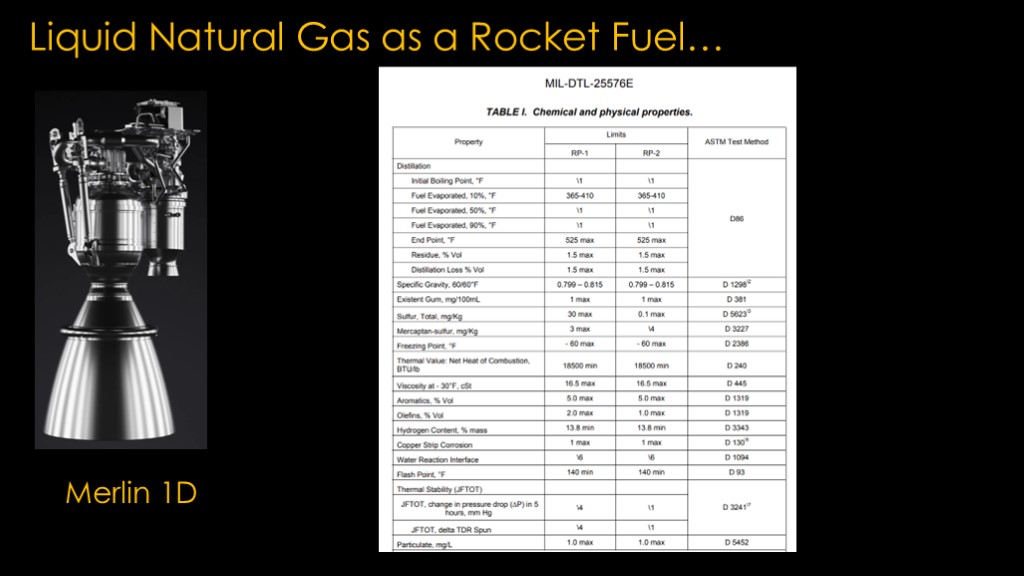
Kerolox engines like the Merlin 1D have a similar issue because the fuel they use is a mix of a bunch of different hydrocarbons. That is why there is a detailed specification that describes the characteristics of the RP-1 fuel that is used, though I expect that rocket companies deal with suppliers that can produce a product that is more precise than required by the specification.
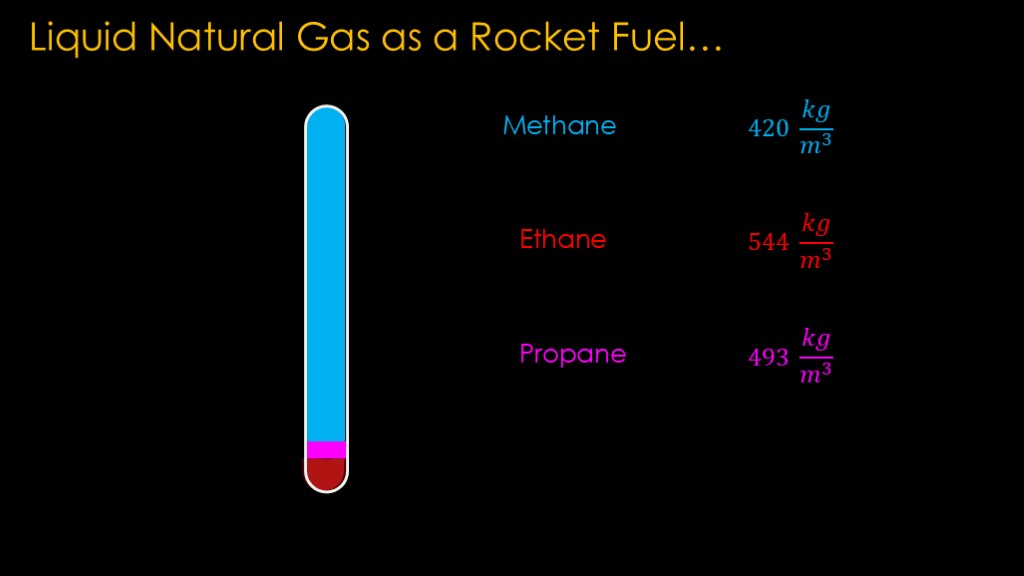
The second problem has to do with density.
Methane has a density of 420 kilograms per cubic meter, ethane has a density of 544, and propane has a density of 493. If we fill our rocket fuel tank with a mix of these three fuels, as it sits on the pad the ethane will sink to the bottom of the tank, there will be a layer of propane on top of it, and the methane will be floating on top of the propane. This is called stratification.
That means our engines get a chunk of ethane at startup, a little blurb of propane, and then the rest is methane. That is going to cause mixture issues.
We therefore want pure liquid methane, or at least something very close to it.
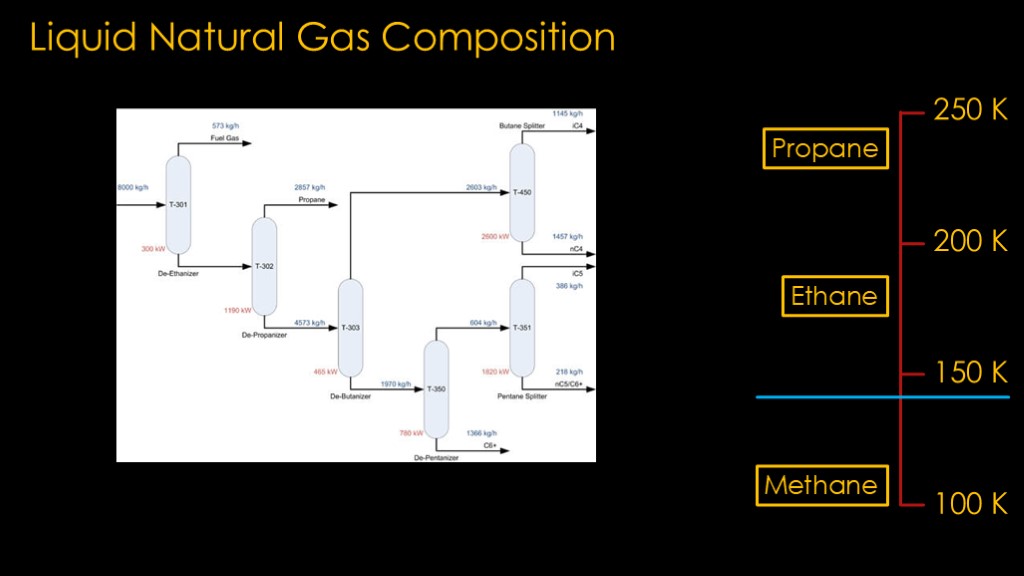
We get that with a similar process to the one used for liquid oxygen; we take advantage of their different liquification temperatures. In this case it's the methane that is the coldest, so we can take the LNG and warm it up enough to boil off the propane and ethane and we're left with mostly methane. The liquid ethane and methane temperatures are significantly different, so we can pick a temperature between the two and our process will work well.
This could be done during the original purification of the well gas or when the natural gas was liquified.
The overall process is a lot more complicated than I'm making it.
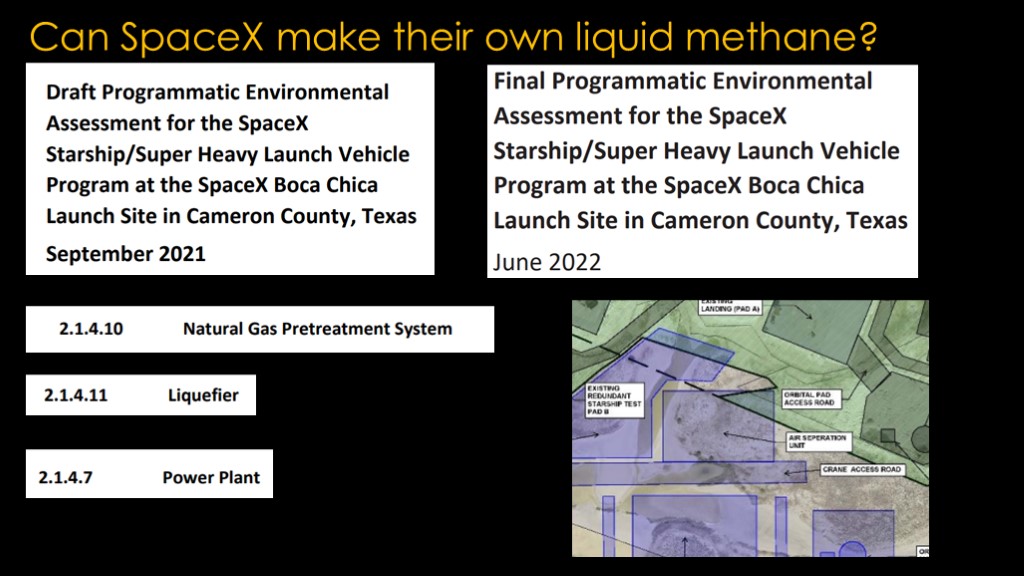
Could SpaceX do this?
The answer is "yes". They could take a natural gas pipeline and build their own liquid methane plant.
In fact, in the original plans for Boca Chica, SpaceX planned to build a natural gas pretreatment system and a liquefier to produce both liquid methane and liquid oxygen.
That would take a lot of power, and they also proposed a natural gas power plant to power those systems. That would nicely give them a way to utilize the ethane and propane that came from the liquid methane creation process.
The natural gas plant and power plant didn't make it into the final Programmatic environmental assessment, and there's no mention of a liquid oxygen plant.
For the time being, it looks like SpaceX is happy with the commercial suppliers. For 2025, their flight rate is unlikely to be more than 25 launches, which is less than 2% of the 4 launches a day we have been looking at, so it's not a big issue right now.
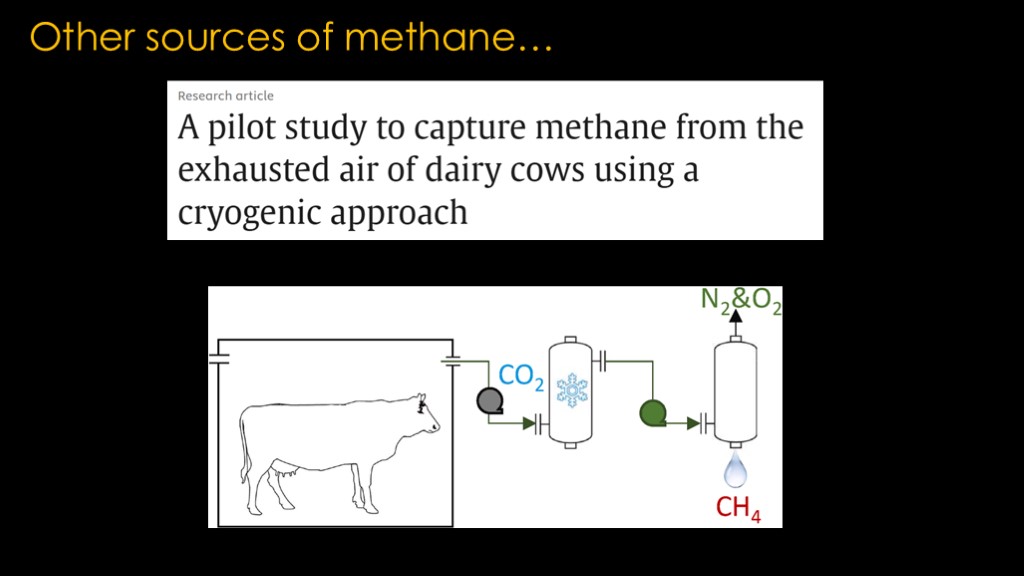
There are other sources of methane. I came across this article, entitled a pilot study to capture methane from the exhausted air of dairy cows using a cryogenic approach.
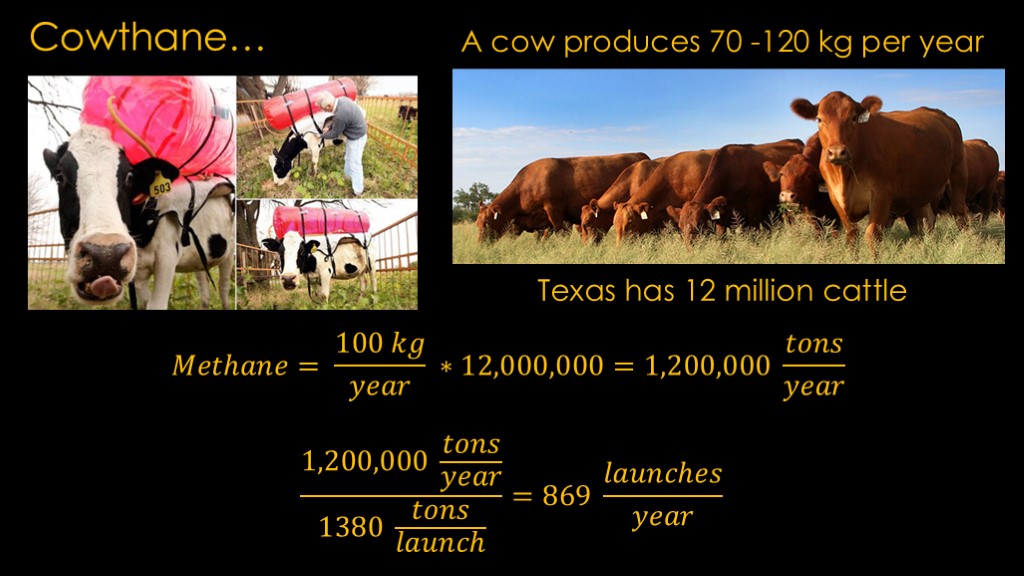
Is there promise in cowthane?
Let's run some numbers...
A cow produces 70 to 120 kilograms of methane per year. Texas has 12 million cattle.
That means the cattle are producing 1.2 million tons per year.
With only 1380 tons of methane required for a launch, that means that cowthane could support 869 launches per year.
Modelling the technology to capture and refine the cowthane is left as an exercise to the reader.
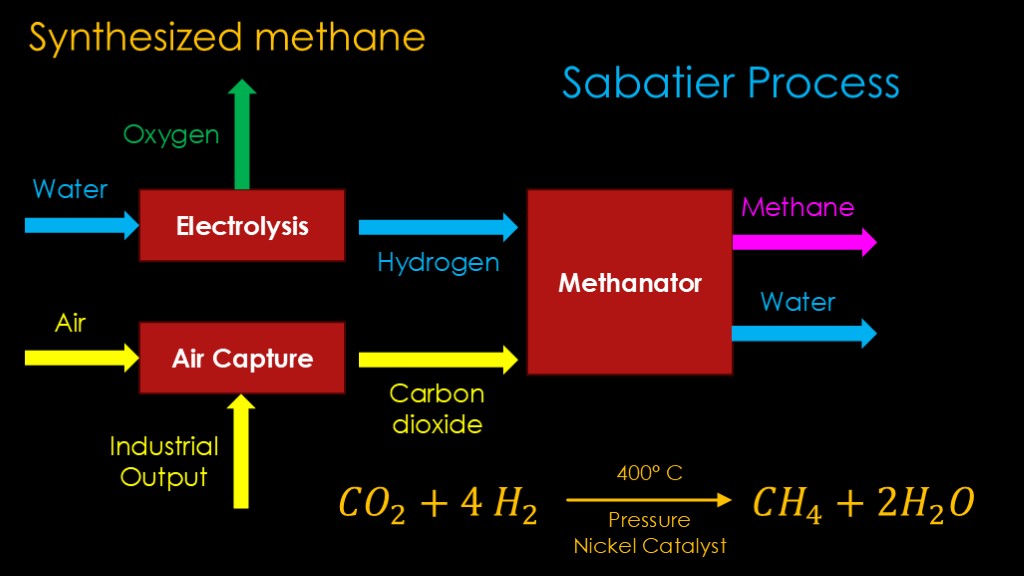
My guess is that you weren't think of cowthane, you were thinking of synthetic methane.
The process is conceptually fairly simple.
You feed purified water into an electrolysis cell which uses electricity to break it apart into oxygen and hydrogen. The oxygen will be liquified to add to our liquid oxygen supply.
You feed air into an air capture system which extracts out the carbon dioxide from the air. You might also use an industrial output of carbon dioxide - perhaps the output of a natural gas power plant - to make it cheaper to get the carbon dioxide.
The hydrogen and carbon dioxide feed into a methanator, which converts the hydrogen and carbon dioxide to methane and water. The water is fed back into the electrolysis step, and the methane is our final product.
For those of you who speak chemistry, here's what the reaction looks like. Starting with carbon dioxide and four hydrogen molecules, heat them to 400 degrees Celsius and pressurize them in the presence of a nickel catalyst, and you will get one methane molecule and two water molecules.
This is know as the Sabatier process.
We can now talk about methane prices.
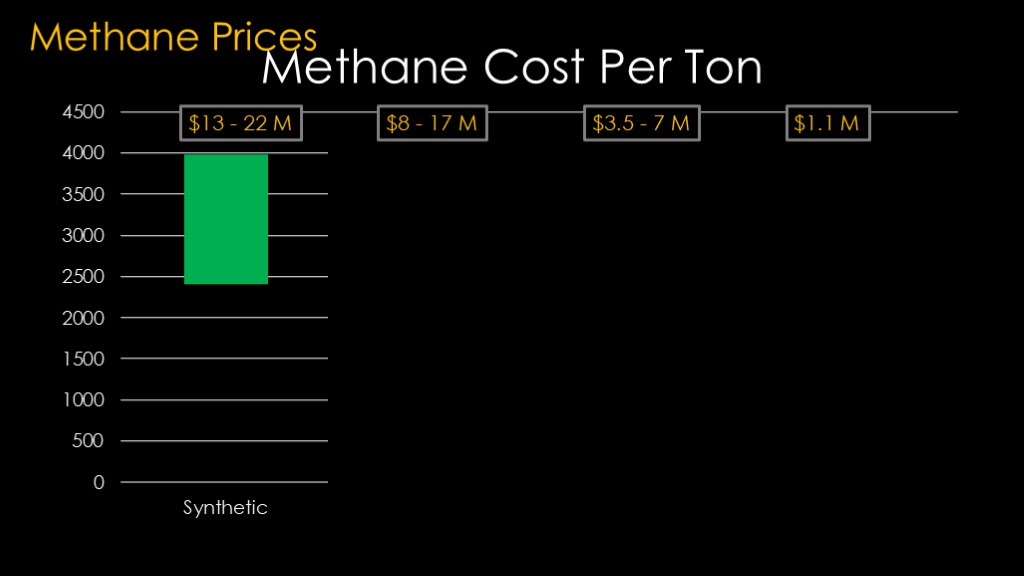
Fully synthetic methane is expensive because the amount of energy required, and it will probably run about $2400 to $4000 per ton. That's $13-22 million for four launches per day.
The synthetic version pulls carbon out of the air and that means you can get carbon credits and other tax breaks. That drops the price down to about $1400 to $3100 per ton, or $8-17 million for 4 launches per day.
The next category is bio methane. This basically means anything that doesn't come from the ground, so landfill methane, methane from manure, and cowthane if it is available. The projected cost for bio methane is $700 - $1300 per ton, or $3.5 to 7 million for 4 launches a day.
The last option is of course purified pipeline gas, which is roughly $200 per ton, or about $1.1 million for four launches. You will have to refine that to get the pure liquid methane that you need, and that will drive up the price.
If you've wondered why SpaceX doesn't synthesize their own methane, this is a pretty good indication why - synthetic methane is much more expensive than getting it from natural gas.
Note that none of these numbers are particularly solid, but they do give you a basic idea of what's going on.

And that's the story on gassin up starship...
SpaceX has so far been content with buying their propellant from commercial companies, and SpaceX has no special expertise in producing either propellant that is used in Starship, so I expect them to continue that arrangement.
I do expect that their suppliers will build appropriate refining plants close to Starship launch pads. Air Liquide already has a plant south of Kennedy Space Center. There are natural gas pipelines near both Kennedy space center and Boca Chica, and both could conceivably be supplied with liquified natural gas by ship.
The big barriers I see are getting enough electricity to run their plants and the time it will take the existing suppliers to expand their production of propellant to support the Starship flight rate.

If you enjoyed this video, you might like this. Just click on the link...
https://www.youtube.com/watch?v=zRgWvvkSvfk